Responsible use of antibiotics
Home » Veterinary medicines » Responsible use of antibiotics
Prudent and responsible use of antibiotics in animals and humans can lower the risk of bacteria becoming resistant. This is particularly important for antibiotics used to treat both people and animals and for antibiotics that are the last line of treatment for critical infections in people.
Antibiotic means any substance with a direct action on bacteria used for treatment or prevention of infections, or infectious diseases.
Unlike antibiotics, antimicrobial is broader term and means any substance with a direct action on microorganisms used for treatment, or prevention of infections, or infectious diseases, including antibiotics, antivirals, antifungals and anti-protozoals.
Antimicrobial resistance means the ability of microorganisms to survive, or to grow in the presence of a concentration of an antimicrobial agent which is usually sufficient to inhibit, or kill microorganisms of the same species. It is the ability of microorganisms, such as bacteria, to develop increasing resistance to antimicrobial product to which they were previously sensitive.
European Medicines Agency (EMA) categorized antibiotics (Categorisation of antibiotics in the European Union) taking into account the need for their use in animals in relation to the risk of antimicrobial resistance to public health.
Antibiotics are classified to following categories:
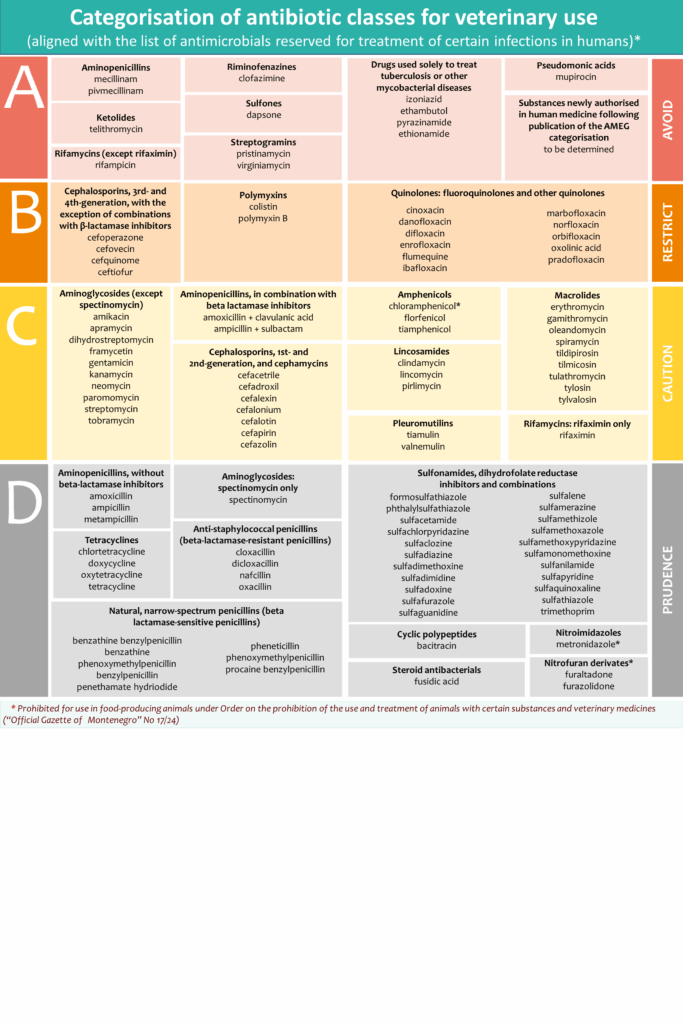
Category A: “Avoid” includes antibiotics that are currently not authorised in veterinary medicine in the European Union (EU), or Montenegro. These medicines should not be used in food-producing animals, while it is only under exceptional circumstances that they may be given to individual companion animals;
Category B: “Restrict” refers to quinolones, 3rd- and 4th-generation cephalosporins and polymyxins. Antibiotics in this category are critically important in human medicine and their use in animals should be restricted to mitigate the risk to public health. They should be considered only when there are no antibiotics in Categories C, or D that could be clinically effective;
Category C: “Caution” covers antibiotics for which alternatives in human medicine generally exist in the EU, but there are no alternatives belonging to Category D available for certain veterinary indications. These antibiotics should only be used when there are no antimicrobial substances in Category D that would be clinically effective.
Category D: “Prudence” includes antibiotics that should be used as first line treatments, whenever possible. These antibiotics can be used in animals in a prudent manner. This means that unnecessary use and long treatment periods should be avoided, and group treatment should be restricted to situations where individual treatment is not feasible.
The categorisation of antibiotic classes for veterinary use in the EU, along with examples of active substances per class, is presented in the infographic available on EMA’s website, and includes routes of administration and types of formulation sorted by their estimated impact on AMR.
Routes of administration and types of formulation are suggested and ranked from the lowest to the highest estimated impact on antibiotic resistance:
- Local individual treatment (e.g. udder injector, eye or ear drops)
- Parenteral individual treatment (intravenously, intramuscularly, subcutaneously)
- Oral individual treatment (i.e. tablets, oral bolus)
- Injectable group medication (metaphylaxis), only if appropriately justified
- Oral group medication via drinking water/milk replacer (metaphylaxis), only if appropriately justified
- Oral group medication via feed or premixes (metaphylaxis), only if appropriately justified
Metaphylaxis means the administration of a medicinal product to a group of animals after a diagnosis of clinical disease in part of the group has been established, with the aim of treating the clinically sick animals and controlling the spread of the disease to animals in close contact and at risk and which may already be subclinically infected.
This categorization can help veterinarians when deciding which antibiotic to prescribe to animals, taking into account other factors such as supporting information in the Summary of Product Characteristics, constraints around use in food-producing species etc.
Brochure on responsible use of antibiotics prepared by CInMED is available in the section Publications.
Ministry of Agriculture, Forestry and Water Management has issued the Order forbidding the treatment the animals using the specific substances and veterinary medicines (“Official Gazette of Montenegro”, No. 017/24 from 27 February 2024).
The provisions of the European Union regulation in the area of forbidding the treatment of animals using the specific substances and veterinary medicines, as well as requirements for the conduct of official controls in this area, have been transposed into this Order. The major innovation compared to the previously valid regulation in this area is forbidding the use of antimicrobial substances and groups of antimicrobial substances in veterinary medicines and in medicated animal food, as well as forbidding the use of human medicines in animals that contain antimicrobial substances or groups of antimicrobial substances intended exclusively for treatment certain infections in humans, in order to preserve their efficacy in human medicine and support the fight against antimicrobial resistance, as a major threat to global health. The list of antimicrobial substances and groups of antimicrobial substances (antibiotics, antivirals and one antiprotozoal medicine) is provided in Annex 1 of the Order.
The Order is available in the section Legislation.
Search register
Here you can search for medicines in our register


Sign up for
Newsletter









